Fibrin Formation Calculator
Enter values and click Calculate to see the impact on fibrin formation and clot stability.
About Fibrin Formation
Fibrinogen is converted to fibrin by thrombin, forming a mesh that stabilizes blood clots. Low levels increase bleeding risk, while high levels may promote thrombosis.
Key Takeaways
- Fibrin is the protein mesh that stabilizes a blood clot.
- Thrombin converts fibrinogen into fibrin during the coagulation cascade.
- Platelets initiate clotting, but fibrin provides the final strength.
- Disorders that affect fibrin formation lead to excessive bleeding or dangerous clots.
- Anticoagulant drugs target the fibrin‑forming steps to prevent thrombosis.
When a vessel wall is injured, the body launches a rapid response to stop the leak. Most people think of platelets as the "clot makers," but the real scaffold that locks everything together is fibrin a fibrous protein that polymerizes into a three‑dimensional network, sealing the wound and giving a clot its tensile strength. Understanding how fibrin works sheds light on why bleeding disorders happen, how anticoagulant drugs act, and what biomarkers clinicians watch for during a heart attack or stroke.
The Biochemistry Behind Fibrin Production
The journey from soluble protein to solid mesh starts with fibrinogen a large plasma protein made by the liver, circulating at roughly 2-4 g/L in healthy adults. When blood vessels are damaged, the enzyme thrombin a serine protease generated from prothrombin by the intrinsic and extrinsic pathways of the coagulation cascade cleaves fibrinogen, removing small peptides called fibrinopeptides A and B. This cleavage exposes binding sites that allow fibrin monomers to link head‑to‑tail, forming long strands that quickly intertwine.
These strands don’t stay loose; they undergo a process called "polymerisation" where they cross‑link, creating a stable, insoluble fibrin clot. The cross‑linking is catalyzed by factor XIIIa, a transglutaminase that locks the network into place, making it resistant to the shear forces of circulating blood.
How Platelets and Fibrin Work Together
Platelets act as the first responders. Upon vessel injury they adhere to exposed collagen and release granules packed with ADP, serotonin, and calcium. This triggers platelet activation and aggregation, forming a temporary "white" clot. However, without fibrin the plug is fragile and can be dislodged.
Once thrombin is generated-primarily on the platelet surface-the fibrinogen‑to‑fibrin conversion begins. The growing fibrin mesh weaves through the platelet plug, anchoring it to the damaged wall and expanding the clot’s size. In essence, platelets start the party, while fibrin builds the house.
Key Steps of the Coagulation Cascade
| Stage | Primary Enzyme | Result |
|---|---|---|
| Initiation (Extrinsic) | Factor VIIa + Tissue Factor | Activation of Factor X to Xa |
| Amplification (Intrinsic) | d>Factor XIa → IXa → VIIIaMore Xa generated, speeding thrombin production | |
| Propagation | Factor Xa + Va (prothrombinase complex) | Conversion of prothrombin to thrombin |
| Fibrin Formation | Thrombin | Fibrinogen → Fibrin + cross‑linking by Factor XIIIa |
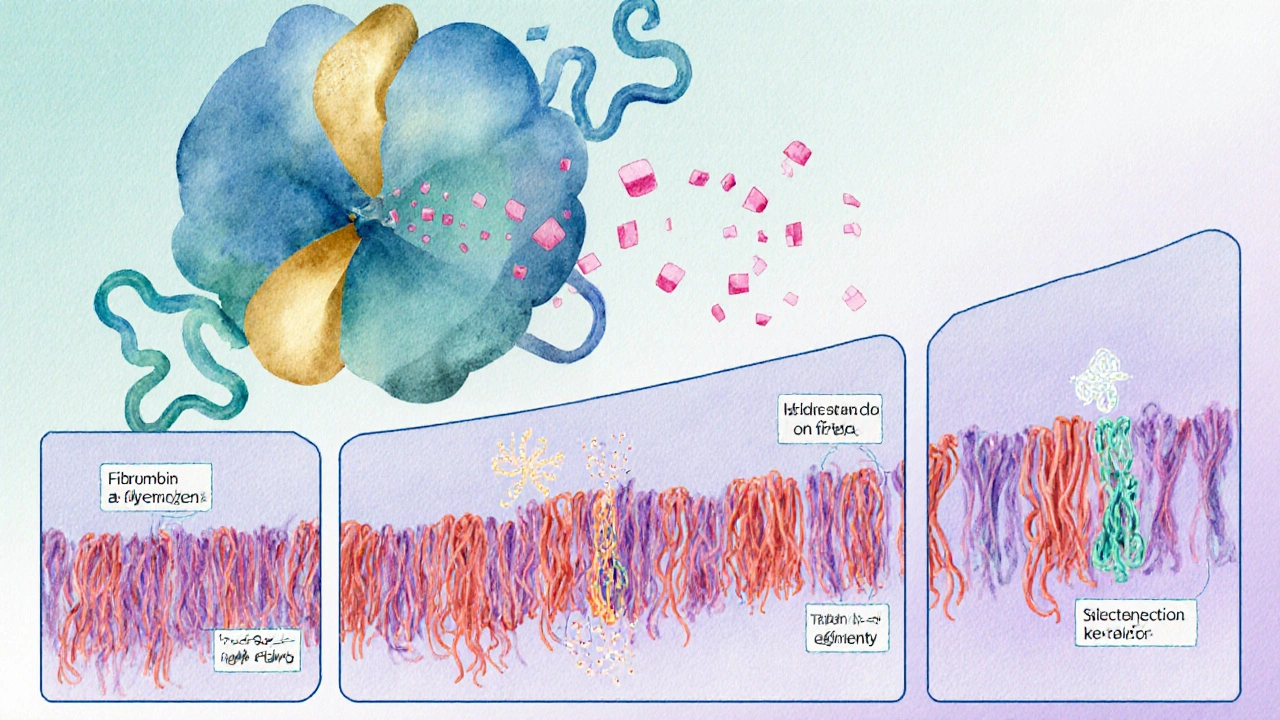
Clinical Relevance: When Fibrin Goes Wrong
Problems can arise at any point in the cascade. If fibrinogen levels drop (a condition called hypofibrinogenemia), the clot lacks enough material to form a sturdy mesh, leading to prolonged bleeding. Conversely, excessive fibrin production can cause pathological clots-thrombosis-in arteries or veins, setting the stage for heart attacks, strokes, or pulmonary embolisms.
One well‑known bleeding disorder is hemophilia a genetic deficiency of clotting factor VIII (Hemophilia A) or IX (Hemophilia B) that impairs thrombin generation and thus fibrin formation. Patients with hemophilia experience spontaneous joint bleeds because their clots never achieve sufficient fibrin density.
On the opposite side, many anticoagulant drugs aim to dampen fibrin production. Direct oral anticoagulants (DOACs) such as apixaban or rivaroxaban inhibit Factor Xa, reducing thrombin formation and consequently fibrin generation. Monitoring D‑dimer levels-a breakdown product of fibrin-helps clinicians gauge how much clotting activity is occurring in the body.
Factors That Influence Fibrin Quality
- Calcium concentration: Required for several steps of the cascade; low calcium (hypocalcemia) slows fibrin formation.
- pH level: Acidic environments reduce thrombin activity, yielding weaker fibrin networks.
- Temperature: Hypothermia impairs enzymatic reactions, delaying fibrin polymerisation.
- Inflammatory mediators: Cytokines like IL‑6 can up‑regulate fibrinogen synthesis, increasing clot risk during chronic inflammation.
Diagnostic Tools Centered on Fibrin
Laboratories assess fibrin-related parameters to diagnose bleeding or clotting disorders:
- Fibrinogen assay: Measures plasma fibrinogen concentration; values < 150mg/dL suggest a bleeding risk.
- Thrombin time: Gauges how quickly thrombin converts fibrinogen to fibrin; prolonged time indicates inhibitor presence or low fibrinogen.
- D‑dimer test: Detects fibrin degradation fragments; elevated levels signal active clot breakdown, common after deep‑vein thrombosis or pulmonary embolism.
Therapeutic Interventions Targeting Fibrin
When fibrin formation is insufficient, clinicians replace it directly. Cryoprecipitate-a plasma‑derived product rich in fibrinogen-boosts fibrin levels in trauma patients. For excessive clotting, fibrinolytic agents like alteplase activate plasmin, which chops fibrin strands and dissolves the clot.
Emerging therapies aim to modulate fibrin structure rather than just block it. Researchers are testing small molecules that produce thinner fibrin fibers, potentially reducing the risk of occlusive clots while preserving hemostasis.
Practical Tips for Managing Fibrin‑Related Risks
- Maintain adequate vitaminK intake (leafy greens) to support synthesis of clotting factors that feed thrombin production.
- Stay hydrated; low plasma volume concentrates clotting proteins and can promote unwanted fibrin deposits.
- If you’re on anticoagulants, keep regular check‑ups for D‑dimer and fibrinogen to avoid over‑ or under‑coagulation.
- Patients with liver disease should monitor fibrinogen levels, as the liver produces most clotting proteins.
In a nutshell, fibrin is the unsung hero that transforms a shaky platelet plug into a robust, life‑saving clot. Whether you’re a patient managing a bleeding disorder, a clinician interpreting lab results, or simply curious about why you stop bleeding, recognizing fibrin’s central role offers a clearer picture of the body’s emergency repair system.
Frequently Asked Questions
What is the difference between fibrinogen and fibrin?
Fibrinogen is a soluble plasma protein that circulates in the blood. When thrombin cleaves it, fibrinogen becomes fibrin, an insoluble strand that forms the mesh of a clot.
Can low fibrinogen cause excessive bleeding?
Yes. Levels below about 150mg/dL reduce the amount of fibrin that can be generated, making clots weak and increasing bleeding time.
Why is D‑dimer used to rule out a pulmonary embolism?
D‑dimer is a fragment released when fibrin is broken down by plasmin. Elevated D‑dimer suggests that clot formation and subsequent breakdown are occurring, a hallmark of PE.
How do anticoagulants affect fibrin formation?
Most anticoagulants inhibit enzymes (like Factor Xa or thrombin) that are essential for converting fibrinogen to fibrin, thereby slowing or preventing clot stabilization.
What lifestyle changes help maintain healthy fibrin levels?
Eating a balanced diet rich in vitaminK, staying well‑hydrated, exercising regularly, and following medical advice on any prescribed blood‑thinners all support normal fibrin dynamics.
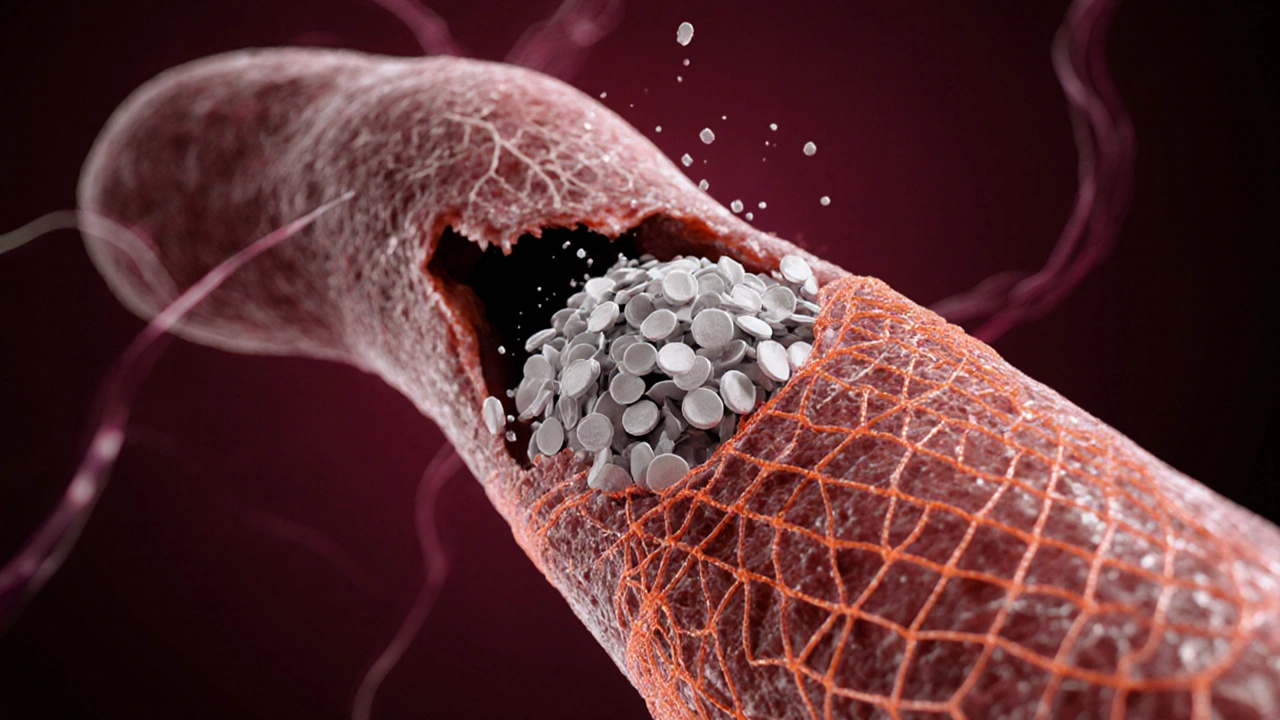
Laura Barney
Wow, that deep dive into fibrin is like a vivid tapestry of biochemistry – the way you described the platelet‑first party and the fibrin house‑building really paints the process in bright colors.
I especially liked the analogy of fibrin being the “unsung hero” that turns a flimsy plug into a sturdy bridge.
Thanks for breaking it down in a way that feels both scientific and story‑like!
Jessica H.
While the article is thorough, the presentation suffers from occasional redundancy, particularly in the sections describing the coagulation cascade where identical enzyme functions are reiterated without providing novel insight. Moreover, the lack of citation to recent peer‑reviewed studies on fibrinogen polymorphisms undermines the authority of the claims. A more disciplined editorial focus would enhance readability and scholarly impact.
Tom Saa
It strikes me that the transformation from fibrinogen to fibrin mirrors the alchemical shift from potential to actuality; a silent promise fulfilled only when the right catalyst, thrombin, intervenes. In that sense, the clot is not merely a physical barrier but a manifestation of latent order emerging from chaos.
John Magnus
The fibrin formation cascade exemplifies a tightly regulated proteolytic network that integrates extracellular cues with intracellular signaling pathways.
Upon vascular injury, tissue factor exposure initiates the extrinsic pathway, leading to factor VIIa activation and subsequent conversion of factor X to Xa.
Factor Xa, in conjunction with its cofactor Va, assembles the prothrombinase complex, catalyzing the generation of thrombin from prothrombin.
Thrombin serves as the pivotal serine protease that cleaves fibrinogen's A and B fibrinopeptides, exposing polymerization sites on the fibrin monomers.
The exposed knobs A and holes a subsequently engage in half‑staggered D‑E interactions, driving the rapid polymer assembly into proto‑fibrils.
Factor XIIIa, activated by thrombin in the presence of calcium ions, cross‑links the γ‑chains of adjacent fibrin strands, cementing the three‑dimensional mesh.
This cross‑linking confers resistance to mechanical shear and proteolytic degradation, thereby stabilizing the clot under circulatory stress.
Dysregulation at any node-be it hypo‑ or hyper‑activity of factor Xa, aberrant thrombin generation, or deficient factor XIII-can tip the hemostatic balance toward bleeding or thrombosis.
Clinically, elevated plasma fibrinogen concentrations, often observed in acute phase responses, correlate with increased viscoelastic clot strength and heightened thrombotic risk.
Conversely, congenital hypofibrinogenemia or acquired consumption coagulopathies manifest as attenuated fibrin polymer density, predisposing patients to hemorrhagic complications.
Therapeutic interventions such as direct factor Xa inhibitors (e.g., apixaban) attenuate thrombin burst, thereby curbing fibrin mesh formation without completely abolishing platelet plug formation.
On the other hand, fibrinolytic agents like tissue‑type plasminogen activator (tPA) catalyze plasmin generation, selectively degrading fibrin fibers to restore vessel patency.
Emerging nanotechnologies aim to modulate fibrin architecture by delivering targeted anti‑cross‑linking molecules that fine‑tune fiber thickness and porosity.
Advanced viscoelastic assays, including rotational thromboelastometry (ROTEM), now provide real‑time quantification of fibrin contribution to clot firmness.
In sum, a granular understanding of each molecular interaction within the fibrin cascade enables precision medicine approaches to both prevent pathological clotting and treat bleeding disorders.
Marc Clarke
Nice overview!
angelica maria villadiego españa
I totally get the feeling you described – it’s fascinating how a cascade of tiny molecular events leads to something as vital as a clot. It really puts into perspective how delicate the balance is inside our bodies.
Ted Whiteman
Honestly, all this hype around fibrin feels a bit overblown; sure, it’s important, but the real star in hemostasis is the platelet, which gets all the applause while fibrin just lurks in the background waiting for a chance to shine.
Dustin Richards
While platelets undoubtedly initiate clot formation, the durability and integrity of the resultant clot depend entirely on the fibrin scaffold; without a robust fibrin network, the platelet plug would dissolve under minimal shear stress.
Vivian Yeong
The article adequately covers the basics, yet it overlooks recent data on fibrinogen isoform variability, which could have added depth to the discussion.
suresh mishra
Good point – studies from 2022 show that γ‑prime fibrinogen variants modulate thrombin binding and may influence clot rigidity, a nuance worth including.
Reynolds Boone
Does anyone have tips on how to practically use the fibrin formation calculator in a clinical setting? I’m curious about reference ranges for different patient populations.
Angelina Wong
When you plug in typical adult values-fibrinogen around 300 mg/dL and thrombin activity near 70%-the calculator flags a low‑risk profile; for pediatric or trauma patients, adjust the thresholds accordingly to capture the heightened coagulation dynamics.