When you’re living with Crohn’s disease or ulcerative colitis, the constant pain, fatigue, and unpredictable flare-ups can make everyday life feel impossible. For many, conventional treatments like steroids or immunomodulators don’t cut it anymore. That’s where IBD biologics come in - targeted drugs that stop the immune system from attacking the gut. These aren’t just another pill. They’re precision tools designed to silence specific parts of the immune response that drive inflammation. And today, there are more options than ever.
What Are IBD Biologics?
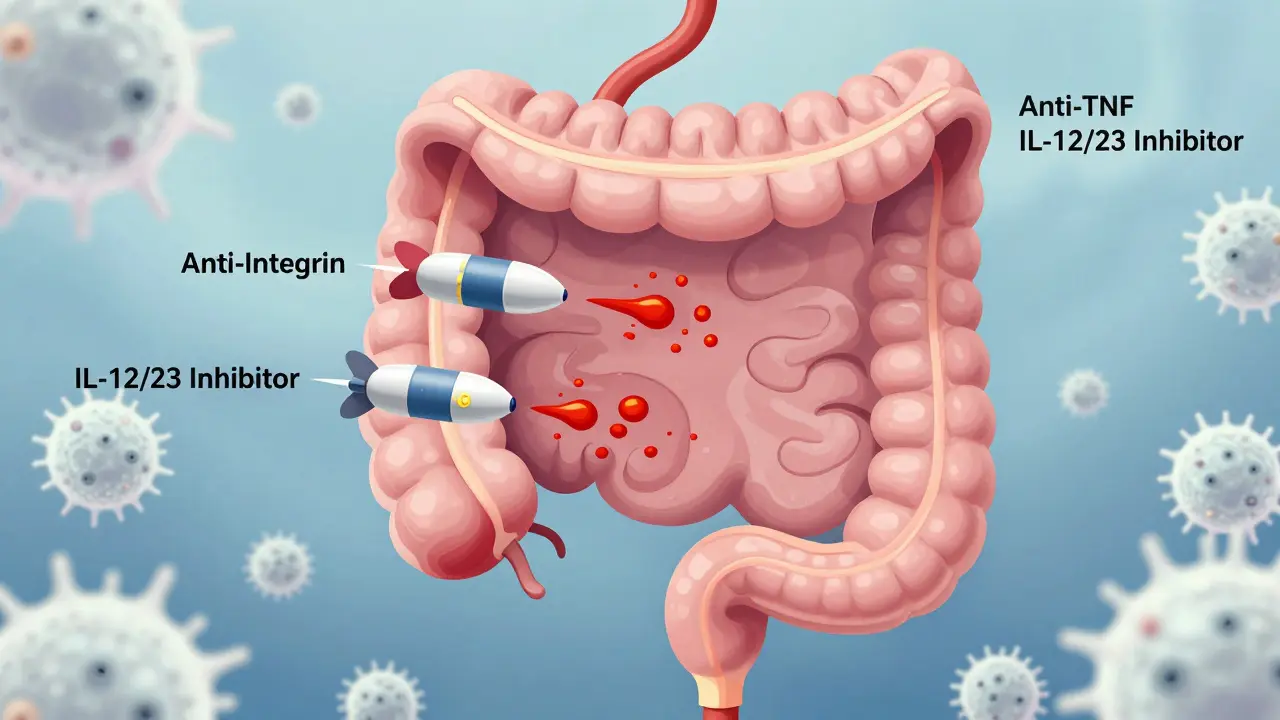
IBD biologics are lab-made proteins that mimic natural molecules in your body. Instead of broadly suppressing your immune system like steroids do, they zero in on one or two troublemakers - like TNF-alpha, integrins, or interleukins - that are overactive in inflammatory bowel disease. Think of them as smart missiles instead of carpet bombs. They’re not new. The first one, infliximab (Remicade), hit the market in 1998. But since then, the toolbox has exploded. Today, there are three main classes: anti-TNF agents, anti-integrins, and IL-12/23 inhibitors. Each works differently, has different side effects, and fits different patient needs.Anti-TNF Agents: The OG Biologics
Anti-TNF drugs were the first to prove that targeting inflammation at the molecular level could change the course of IBD. They block tumor necrosis factor-alpha (TNF-alpha), a protein that acts like a flare signal for immune cells in the gut. The big names here are:- Infliximab (Remicade) - given as an IV infusion every 8 weeks after three initial doses
- Adalimumab (Humira) - a self-injected shot every other week
- Golimumab (Simponi) - monthly injection
- Certolizumab pegol (Cimzia) - every 2-4 weeks, also self-injected
Anti-Integrin Therapies: Gut-Selective and Safer
If you’re worried about infections or have other autoimmune conditions like multiple sclerosis, anti-integrin drugs might be a better fit. They don’t mess with your whole immune system. Instead, they block white blood cells from entering the gut lining - like putting up a barrier at the front door. The only two approved for IBD are:- Vedolizumab (Entyvio) - IV infusion every 8 weeks
- Natalizumab (Tysabri) - approved for MS, not IBD, due to PML risk

IL-12/23 and IL-23 Inhibitors: The New Kids on the Block
The newest wave of biologics targets interleukins - signaling proteins that help immune cells talk to each other. Ustekinumab (Stelara) blocks both IL-12 and IL-23. The newer ones - risankizumab (Skyrizi) and mirikizumab (Omvoh) - focus only on IL-23, which appears to be the bigger driver of chronic gut inflammation.- Ustekinumab (Stelara) - injected under the skin every 8 or 12 weeks, depending on weight
- Risankizumab (Skyrizi) - approved for Crohn’s in 2019, then for ulcerative colitis in June 2024
- Mirikizumab (Omvoh) - approved for UC in 2022, now being tested for Crohn’s
Which One Is Right for You?
There’s no single best biologic. The right choice depends on your disease, your life, and your priorities.- Need fast results? Infliximab or adalimumab usually work within weeks.
- Worried about infections? Vedolizumab or IL-23 inhibitors are safer.
- Want to avoid clinics? Adalimumab, ustekinumab, and risankizumab are self-injected.
- Have psoriasis? Vedolizumab is preferred - anti-TNFs can make it worse.
- Cost is a problem? Biosimilars cut anti-TNF prices by up to 30%. Manufacturer programs often cover 95% of costs.
What About Cost and Access?
These drugs aren’t cheap. A single dose of vedolizumab runs about $5,500. Ustekinumab? Around $7,200. Even with insurance, copays can hit $1,000 a month. That’s why patient assistance programs are critical. Janssen’s Janssen CarePath, for example, helps eligible patients pay $0-$5 per infusion. Insurance approvals can be a nightmare. Many require trying oral meds first. Others demand proof of failed treatment. That delay can mean months of unnecessary suffering. The market is growing fast. IBD biologics hit $18.7 billion in 2023 and are expected to hit $32.4 billion by 2030. Anti-TNFs still dominate with 65% of sales, but IL-23 inhibitors are growing 25% a year. By 2028, they could take 30% of the market.What You Need to Know Before Starting
Starting a biologic isn’t just about picking a drug. It’s about preparing for the long haul.- Vaccines first. Get all your shots - flu, pneumonia, shingles - before starting. Live vaccines (like MMR) are off-limits once you’re on biologics.
- Screen for TB. A skin test or blood test is required for all anti-TNFs.
- Know the signs of infection. Fever, chills, cough, or new skin sores? Call your doctor. Don’t wait.
- Track your symptoms. Apps like MyTherapy help patients stick to schedules and spot flare patterns.
- Expect side effects. Infusion reactions (itching, dizziness) happen in up to 10% of cases. Injection site pain? Common with self-injectables.
The Future of IBD Treatment
We’re moving toward personalized care. Doctors are starting to use blood tests and stool markers to predict who will respond to which drug. Trials like RHEA and VEGA are comparing biologics head-to-head for the first time - something we’ve lacked for years. The next wave includes drugs like etrolizumab (an anti-integrin in phase 3) and new oral therapies that could replace injections entirely. But for now, biologics remain the gold standard for moderate-to-severe IBD. The message is clear: you’re not stuck with flares. There are powerful tools available. The trick is matching the right one to your life - not just your disease.How long does it take for IBD biologics to work?
It varies by drug. Anti-TNF agents like infliximab and adalimumab usually start working in 2-4 weeks. Vedolizumab takes longer - 6 to 10 weeks for noticeable improvement. IL-23 inhibitors like risankizumab may take 8-12 weeks to show full effects. Some patients feel better sooner, but full remission often takes months.
Can I switch from one biologic to another?
Yes, switching is common. About 30% of patients need to change biologics within five years due to loss of effectiveness or side effects. Many switch from anti-TNFs to vedolizumab or IL-23 inhibitors for better safety. Your doctor will monitor your response and adjust based on symptoms, lab tests, and endoscopy results.
Are biosimilars as good as the original biologics?
Yes. Biosimilars like Inflectra (for infliximab) and Cyltezo (for adalimumab) are approved by the FDA as highly similar to the original drugs, with no clinically meaningful differences in safety or effectiveness. They’ve been used for years in Europe and the U.S. with strong real-world data supporting their use. Cost savings are significant - up to 30% lower.
Do I need to stop biologics before surgery?
It depends. For elective surgery, many doctors pause anti-TNFs for 4-8 weeks before and after to reduce infection risk. Vedolizumab and IL-23 inhibitors are often continued, as they carry lower surgical risks. Always discuss timing with your gastroenterologist and surgeon - decisions are individualized based on disease activity and surgical urgency.
Can I drink alcohol while on IBD biologics?
Moderate alcohol is usually fine, but heavy drinking increases liver stress and may worsen IBD symptoms. Since biologics can affect liver function, your doctor may monitor liver enzymes. Avoid alcohol if you’re also taking methotrexate or azathioprine. Always check with your care team - individual risks vary.
What are the biggest risks of IBD biologics?
The biggest risks are serious infections (like TB, pneumonia, or sepsis), especially with anti-TNF drugs. There’s also a small increased risk of lymphoma and skin cancer. Natalizumab carries a rare but deadly risk of PML (brain infection), so it’s not used for IBD. IL-23 inhibitors have the safest profile overall. Regular check-ups and screenings are essential.
Will I have to take biologics forever?
Most patients stay on biologics long-term to maintain remission. Stopping often leads to flare-ups - and sometimes the drug won’t work as well if restarted. Some patients in deep, sustained remission may try tapering under close supervision, but that’s rare. The goal is to control the disease, not cure it - and biologics are the most effective tool we have for that.
Lisa Rodriguez
I switched from Humira to Entyvio last year and honestly? Best decision ever. No more injection site pain and zero infections. Sure, it took 8 weeks to kick in but I’d wait 8 months if it meant not being stuck in the bathroom all day. 🙌
Bryan Coleman
just started stelara last month and already feel like a new person. no more fatigue by 3pm. also no more monthly infusions which is a huge win for my work schedule.
Aditya Gupta
India has access to biosimilars now and they’re life changing. My cousin got Inflectra for half the price. Same results. Why pay full price when science says it’s the same?
Nidhi Rajpara
I must emphasize: before initiating any biologic, comprehensive screening for latent tuberculosis is mandatory. Failure to comply may result in disseminated disease with fatal outcomes.
Chris & Kara Cutler
RISANKIZUMAB JUST GOT APPROVED FOR UC??!! 🤯 I’ve been waiting for this since 2023. My GI just called me yesterday to schedule my first dose. I’m crying happy tears 😭💖
Donna Macaranas
I’ve been on Cimzia for 3 years. It’s not perfect but it’s mine. I don’t talk about it much but I’m grateful every day that I can play with my kids without needing a heating pad.
Rachel Liew
I was so scared to start a biologic. Thought I’d turn into a zombie or get cancer. But my doctor explained it all and now I’m in remission. You’re not alone. Just talk to someone.
Nicki Aries
Let’s be real-anti-TNFs are the OGs, but they’re also the overbearing ex who still texts you at 2 a.m. I switched to Skyrizi and now my body isn’t constantly screaming for mercy. Thank you, science.
Ed Di Cristofaro
People who say biosimilars are ‘just as good’ are either pharma shills or haven’t read the real-world data. My cousin lost response after 6 months on Inflectra. Original Remicade worked fine. Don’t be fooled.
vivian papadatu
As someone who moved from the Philippines to the U.S., I was shocked at how expensive these drugs are here. Back home, we use methotrexate and hope. I’m so grateful for access-but it’s still a battle. If you’re lucky enough to be on one, don’t take it for granted.
Melissa Melville
So let me get this straight... we spend $7,000 a month on a drug that stops your immune system from attacking your gut... but the FDA won’t approve a pill that stops you from eating gluten? 🤦♀️