Immunosuppressant Risk Calculator
This tool estimates your risk of infection complications based on your immunosuppressant medication, age, smoking status, and other factors. The article explains why understanding these risks is critical for safe treatment.
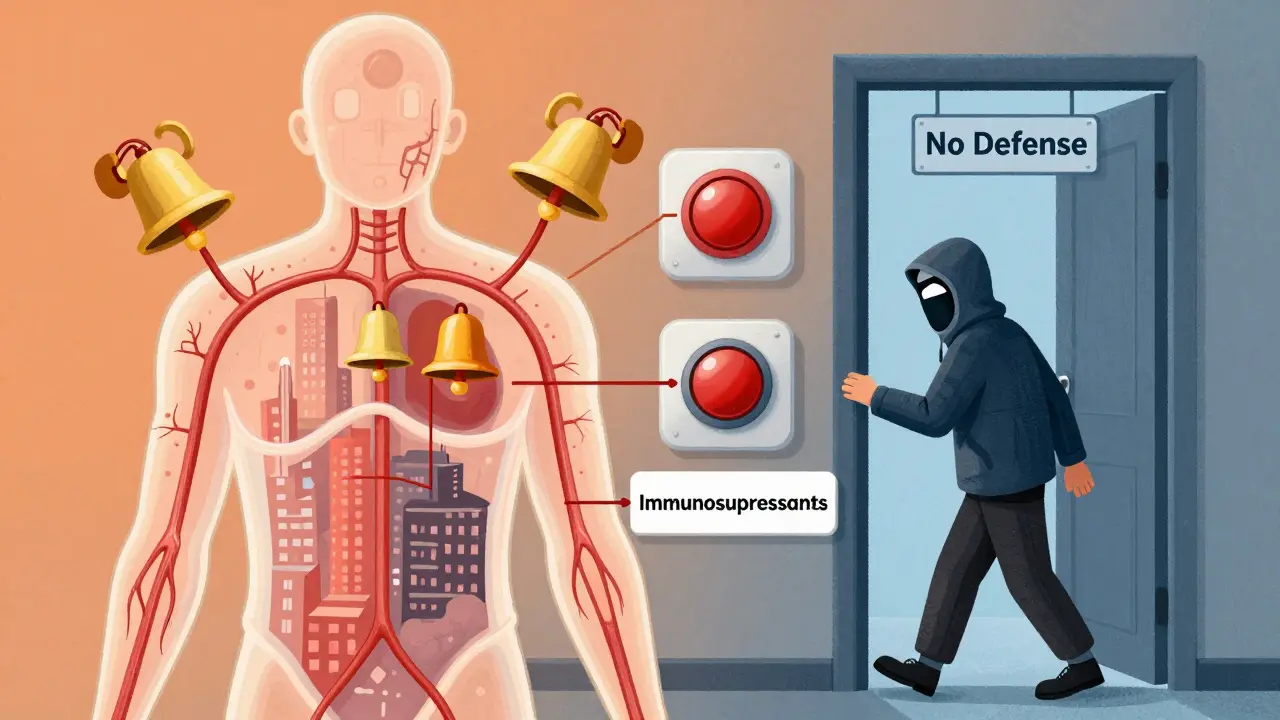
When you’re managing an autoimmune disorder like rheumatoid arthritis, lupus, or Crohn’s disease, the goal is simple: stop your body from attacking itself. But the drugs that make that possible - immunosuppressants - come with a hidden cost. They don’t just quiet the bad immune response. They also turn down the volume on your body’s entire defense system. That’s where the real danger lies.
What Happens When Your Immune System Is Turned Down
Immunosuppressive medications work by targeting specific parts of your immune system. Some block signaling molecules like TNF-alpha. Others wipe out B-cells or stop T-cells from multiplying. The result? Less inflammation. Fewer flare-ups. But also, less ability to fight off infections. Think of it like turning off the alarm system in your house. It stops the false alarms (autoimmune attacks), but now a real burglar (infection) can walk right in. That’s why patients on these drugs are more likely to get pneumonia, urinary tract infections, or even rare illnesses like tuberculosis or fungal infections. The CDC estimates that 5 million Americans are on some form of immunosuppressive therapy - and that number keeps growing every year.The Big Five Drug Classes and Their Hidden Risks
Not all immunosuppressants are the same. Each class has its own fingerprint of side effects. Knowing the difference isn’t just academic - it can save your life. Corticosteroids like prednisone are fast-acting and widely used. But they’re also the most broadly suppressive. If you’re taking more than 20 mg a day for over two weeks, your risk of serious infections jumps significantly. Even after you stop, your immune system can stay weak for weeks. Many patients don’t realize this. They feel better, so they stop monitoring. Then they get sick - and it’s bad. Biologics like Humira, Remicade, and Rituxan are targeted. They don’t hit everything. But they can hit hard. Rituximab, for example, wipes out B-cells. Those cells don’t come back for months. During that time, your body can’t respond to vaccines or new infections. One patient on Reddit described getting shingles four months after their last infusion - even though they’d been vaccinated. Their doctor didn’t warn them about the six-month window of vulnerability. JAK inhibitors like Xeljanz and Olumiant are newer. They’re oral pills, which makes them convenient. But they come with unique risks. Studies show they increase the chance of blood clots, especially in people over 50 or with a history of smoking. The FDA added a black box warning for this in 2021. They also raise the risk of lymphoma and lung cancer in older patients. And despite being called "targeted," they still cause more herpes zoster (shingles) outbreaks than TNF blockers. Calcineurin inhibitors like cyclosporine and tacrolimus are common in transplant patients, but also used for severe psoriasis and lupus. Their biggest problem? Kidney damage. Up to 40% of long-term users develop some level of kidney impairment. That’s not a side effect you can ignore. Methotrexate is the quiet outlier. At low doses (25 mg per week or less), it’s surprisingly safe. It only raises infection risk by about 20% compared to the general population. Many doctors still use it as a first-line treatment for good reason. It’s cheap, well-studied, and doesn’t wipe out your immune system like the others.Who’s Most at Risk - And Why
Not everyone on immunosuppressants gets sick. But some people are far more vulnerable. Age matters. People over 65 are at much higher risk for serious infections and cancers. Smoking? That doubles your lung cancer risk on JAK inhibitors. Diabetes? That makes you more likely to get fungal infections. Obesity? Slows healing and increases surgical complications. And then there’s timing. One of the biggest mistakes doctors make is starting immunosuppressants without checking vaccine status. If you’re about to begin Rituximab, you need your flu shot, pneumonia shot, and shingles vaccine - at least four weeks before. After you start, it’s too late. Your body won’t respond. A 2022 study found that 68% of serious infections in these patients could have been prevented with proper vaccination timing. That’s not a small number. That’s nearly seven in ten.Monitoring Isn’t Optional - It’s Lifesaving
You can’t just take the pill and hope for the best. Regular checks aren’t just paperwork. They’re your safety net. Patients on B-cell depleting drugs like Rituximab need immunoglobulin levels checked every three months. Those on JAK inhibitors need annual testing for varicella zoster antibodies. Anyone on corticosteroids over 20 mg/day needs monthly blood counts and twice-yearly TB skin tests. And yet, a 2023 study from the American College of Rheumatology found that 72% of serious complications happened because of poor monitoring - not because the drugs were inherently dangerous. In other words, the problem isn’t always the medication. It’s the lack of follow-up. Primary care doctors often don’t have the training to manage this. It takes rheumatology fellows 12 months to become proficient. For a regular doctor? It can take 18 months. That’s why many patients end up falling through the cracks.What Patients Are Saying - And What It Means
Real people are sharing their stories online. On PatientsLikeMe, one user with rheumatoid arthritis switched from methotrexate to a biologic after a bad flare. Six months later, they got a severe staph infection that landed them in the hospital. They didn’t know their immune system was this vulnerable. Another user, a nurse with lupus, started taking a JAK inhibitor. Within months, she got shingles - twice. She now checks her VZV titers every six months. She’s not taking any chances. Drug reviews on Drugs.com show hydroxychloroquine with a 7.8 out of 10 safety rating. Biologics? 6.2. JAK inhibitors? 5.9. That’s not just opinion. That’s lived experience. The Arthritis Foundation’s 2022 survey of over 3,200 patients found that 42% stopped biologics because they were afraid of getting sick. Another 28% had been hospitalized due to an infection linked to their treatment. These aren’t rare cases. They’re the norm.What’s Changing - And What’s Next
The field is waking up. In 2023, the FDA required mandatory risk education for all doctors prescribing JAK inhibitors. Medicare now demands proof of vaccination and infection prevention plans before approving biologics. Insurance companies aren’t just paying for the drug - they’re paying for the safety plan around it. Newer drugs are being designed to be more selective. Instead of shutting down entire immune pathways, they’re targeting just the overactive cells. The NIH is funding research to find biomarkers that predict who’s most likely to get infected. Early results using CD4+ T-cell analysis look promising. At the Mayo Clinic, an AI tool that analyzes electronic health records reduced serious infections by 22% in a pilot study. Imagine a system that tells your doctor: "This patient has low IgG levels, hasn’t had a pneumonia shot, and is on high-dose steroids - they need a flu shot and a referral to infectious disease now." That’s the future. And it’s coming fast.What You Should Do Right Now
If you’re on immunosuppressants - or thinking about starting - here’s what to ask your doctor:- Which class of drug am I on, and what are its specific risks?
- Have I had all my vaccines - and were they given at least four weeks before starting?
- What blood tests do I need, and how often?
- Am I at higher risk because of my age, smoking, or other conditions?
- What should I do if I get a fever, cough, or unusual rash?

Hydroxychloroquine: The Quiet Safe Option
For mild autoimmune conditions - like early lupus or mild rheumatoid arthritis - hydroxychloroquine is often the best choice. It doesn’t cause major immunosuppression. It doesn’t increase infection risk significantly. And it’s been used safely for over 70 years. It’s not a miracle drug. It won’t stop a severe flare. But for many people, it’s enough. And the safety profile? It’s unmatched. If your disease is stable, why take a bigger risk than you need to?When to Consider Stopping
Sometimes, the best treatment is no treatment. If you’ve been in remission for two years, your doctor might suggest tapering off. Not everyone can do it. But some can. And for those who can, the benefits are huge: no more monthly blood draws, no more fear of infection, no more worrying about cancer risk. The key? Do it slowly. And under supervision. Stopping cold turkey can cause a rebound flare. But a careful, monitored withdrawal? That’s a win.Final Thought: Control, Not Cancellation
Immunosuppressants aren’t the enemy. They’ve given people their lives back. But they’re not harmless. They’re powerful tools - and like any powerful tool, they demand respect. The goal isn’t to avoid them. It’s to use them wisely. To know your risks. To stay ahead of complications. To ask the hard questions. And to never assume that feeling better means you’re safe. Your immune system isn’t just fighting your disease. It’s fighting for you every day. Don’t let the medicine silence it completely.Can I still get vaccinated while on immunosuppressants?
Yes - but timing matters. Live vaccines (like MMR, shingles, and nasal flu) are unsafe once you start immunosuppressants. Inactivated vaccines (flu shot, pneumonia, COVID-19) are safe but less effective. The best time to get them is at least four weeks before starting treatment. For drugs like Rituximab, wait until your B-cells return - often 6 to 12 months after your last dose - before getting new vaccines.
Do all immunosuppressants increase cancer risk?
Not equally. JAK inhibitors have a clear link to lymphoma and lung cancer, especially in older patients who smoke. Biologics like Rituximab slightly increase skin cancer risk. Corticosteroids may raise risk with long-term use. Methotrexate and hydroxychloroquine show no significant increase in cancer rates. Your doctor should assess your personal risk based on age, smoking, family history, and drug type.
How do I know if I have an infection while on these drugs?
Symptoms can be mild or hidden. A fever as low as 100.4°F (38°C) can be a red flag. So can unexplained fatigue, a cough that won’t go away, or a rash that’s spreading. Diarrhea, joint pain, or confusion can also signal infection. Don’t wait for a high fever. If you feel off, call your doctor immediately - even if you think it’s "just a cold."
Is there a safer alternative to biologics?
For mild to moderate disease, methotrexate and hydroxychloroquine are often safer. Sulfasalazine is another option, especially for gut-related autoimmune conditions. They don’t work as fast or as strongly as biologics, but they’re much lower risk. Many patients stay on them long-term without serious complications. Talk to your rheumatologist about whether your condition can be managed without a biologic.
How often should I get blood tests?
It depends on the drug. For methotrexate: monthly CBC and liver enzymes. For JAK inhibitors: CBC and liver enzymes every 4-8 weeks for the first 6 months, then every 3 months. For biologics like Rituximab: immunoglobulin levels every 3 months. For corticosteroids over 20 mg/day: monthly CBC and kidney function. Never skip these tests. They catch problems before they become emergencies.
Chris Clark
I was on Humira for 3 years. Got shingles at 32. Doc said 'it happens' and handed me antivirals. No one told me my immune system was basically on vacation. Now I'm off it and just on methotrexate. Still have RA but at least I don't feel like a walking petri dish.
William Storrs
You're not alone. I had a friend who got a fungal lung infection from prednisone. They thought it was just a bad cold. By the time they went to the ER, they needed oxygen. Don't ignore a fever. Even 99.8. Call your rheum. Seriously.
Dominic Suyo
The entire pharmaceutical-industrial complex is built on selling you a slow-motion suicide pill wrapped in a 'miracle drug' bow. They don't care if you live or die - they care if you refill your prescription. JAK inhibitors? FDA black box warning? Oh, they'll still push them. Because $$$.
Janelle Moore
I know someone who got cancer on JAK inhibitors. They said it was 'statistically rare.' But what if you're the one? What if you're the 0.7%? I don't trust any doctor who doesn't hand you a printed list of every possible death you might experience before breakfast.
Aadil Munshi
Funny how we treat immunosuppressants like they're magic wands. We're basically asking our bodies to commit suicide to win a war we didn't start. Maybe the real problem isn't the drugs - it's that we keep trying to fix symptoms instead of asking why the body turned on itself in the first place. Just a thought.
Monte Pareek
Listen. If you're on anything stronger than hydroxychloroquine and you haven't had your pneumonia shot, your flu shot, your shingles shot, and your COVID booster - you're playing Russian roulette with your lungs. And if your doctor hasn't scheduled your quarterly IgG levels or your TB skin test? Find a new doctor. This isn't optional. This is survival 101. I've seen too many people lose their lives because they thought 'feeling better' meant 'safe'. It doesn't. Never has. Never will.
mark shortus
I got a staph infection from a tiny cut on my finger while on rituximab. Ended up in the ICU. They said 'it's rare.' I said 'it happened to me.' Now I carry a medical alert card that says 'IMMUNOSUPPRESSED - DO NOT DELAY TREATMENT.' And yes, I know it's dramatic. But I'm alive because I was dramatic.
Carolyn Benson
It's not just the drugs. It's the system. Doctors are overworked. They don't have time to explain the 17 possible ways you can die on these meds. Insurance won't pay for the extra labs. And patients? We're told to 'trust the process.' But trust who? The same people who told us Vioxx was safe? I'm not trusting anyone anymore.
James Stearns
The notion that immunosuppression is a 'necessary evil' is a profound mischaracterization. It is, in fact, a calculated trade-off - one that is often inadequately communicated to patients who are, by virtue of their clinical vulnerability, rendered epistemologically subordinate to the medical establishment. The onus, therefore, must be placed upon the clinician to ensure informed consent is not merely a formality, but a rigorous, documented, and repeated dialogue.
Henry Marcus
They're lying. All of them. The FDA, the CDC, the doctors - they know JAK inhibitors cause blood clots and cancer. But if they admit it, the drug companies lose billions. So they bury the data, push the pills, and tell you to 'take it with food.' I've seen the internal emails. It's not a side effect. It's a feature. For their profit.